Heat Engine
Any practical machine which converts heat into mechanical work is called a heat engine. Heat engines in their operation absorbs heat at Higher temperature, convert part of it into mechanical work, and reject the remaining heat at a low temperature. In this process, a workin substance is used. In steam engines, the working substance is water vapour, and in all gas engines the working substance is a combustible mixture of gases.
The working substance goes through some processes that involes change of pressure(P), Volume(V) and temperature, and then returns to the initial state. This is called as one cycle of operation, since it is a cyclic process.
Heat engines were from long time but were only made useful at the time of industrial revolution in 18th century.
Carnot's Ideal Heat Engine
French engineer Sadi Carnot conceived a theoretical engine in 1824. The engine is not practically possible. It has maximum efficiency. It is an ideal heat engine.
It has following parts:
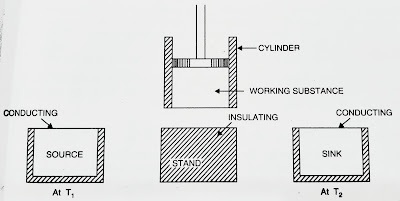
- A cylinder which have perfectly non-conducting walls, a perfectly conducting base, with a perfectly non-conducting piston which moves without friction in the cylinder.
- The cylinder contains a perfect gas.
- Source : A reservoir maintained at a constant temperature T1. It has infinite thermal capacity. Any amount of heat can be drawn from it , and still the temperature will not change.
- Insulating stand : A perfectly non-conducting platform acts as a stand for adiabatic processes.
- Sink : A reservoir maintained at a constant lower temperature T2(T2<T1). Engine can reject any amount of heat to it, the temperature of sink would still remain constant. It also has infinte thermal capacity.
Some important terms-
1) Quasi-Static Process-
A Quasi-Static process is a process carried out very very slowly so that the system under consideration remains at all times arbitrarily close to the equilibrium state. The thermodynamic processes should be Quasi-Static for the P-V diagram to be meaningful.
2) Cyclic Process-
A cyclic process is a thermodynamic process in which the system returns to its initial state after undergoing a series of changes.
It may be a closed cycle or an open cycle.
3) Reversible Process-
A reversible process is that which can be retraced in the opposite direction, so that the working substance passes through exactly the same conditions as it does in the direct process.
In other words it means that in a step where heat is absorbed in the direct process, it is given out in the reverse process and vice versa.
4) Isothermal Process-
The process in which the temperature of the system remains constant is called a isothermal process.
Also by Boyle's law at constant temperature P(pressure) is inversely proportional to V(volume).
5) Adiabatic process-
The process in which no heat transfer takes place. The heat neither transfer inside nor outside the system.
Carnot's Cycle
It is a reversible cyclic process. This cycle consists of isothermal and adiabatic curves on a P-V diagram and is known as Carnot's cycle.
1) Isothermal expansion-
Suppose the cylinder contains one mole of perfect gas. Let the initial temperature of the gas in the cylinder be T1 K. Let the temperature of heat source is also T1 K.
The cylinder is placed on the source, so that the gas absorbs heat at a constant temperature T1. Since the gas absorbs heat from source at constant temperature it undergoes isothermal expansion. It is an quasi-static expansion.
Let the initial state of the working substance be A(P1,V1,T1). And the final state of the working substance during this isothermal expansion be B(P2,V2,T1). This can be expressed in a P-V graph as the curve AB at constant temperature T1.
if the gas absorbs Q1 heat from the source at T1 and does work W1. Since it is an isothermal process
ΔU = 0
Therefore, by first law of thermodynamics
Q1 = W1
Hence,
2) Adiabatic expansion-
The cylinder is now removed from the source and is placed on the insulating stand. Since the stand is insulating, the processes that will happen , will happen without the exchanging of heat.
The gas is allowed to expand slowly. The expansion is called slow adiabatic expansion. The expansion or the work done by the gas is done at the expense of its internal energy, which will decrease the temperature from T1 to T2. Where T2 is the temperature of the sink.
The initial state of the substance is B(P2,V2,T1) and let the final state at the end of this process be C(P3,V3,T2). If the work in this process be W2
3) Isothermal Compression-
Now the cylinder is removed from the insulating stand and is placed on the sink which is at the temperature T2. In this process the gas is compressed. The work is done on the gas. The gas rejects the heat to the sink, hence the temperature of the gas remains constant. Thus the gas undergoes isothermal compression at constant temperature T2 and rejects some heat to the sink.
This process can be represented by the isothermal curve CD. Where C(P3,V3,T2) to the state D(P4,V4,T2). Let the heat rejected by the gas be Q2 to the sink at T2 and work W3 is done on the gas is given by
4) Adiabatic Compression-
The cylinder is now removed from sink and again kept on the insulating stand. Again because the stand is insulating, the process will be adiabatic. The gas will compress, and the temperature will rise. Adiabatic Compression continues till the original condition arrives.
Thus, this adiabatic process can be represented by a adiabatic curve DA. Where D(P4,V4,T2) is the initial state and A(P1,V1,T1) be the final state in this process.
In this process if the work done on the gas is W4 and is given by
Efficiency of heat engine
The efficiency of the heat engine is the ratio of quantity of heat converted into work(useful output) per cycle to the total amount of heat absorbed per cycle.
It T1=T2 i.e. the temperature of the source is equal to the temperature of sink, then efficiency is zero i.e. the engine does not work.


.jpg)
.jpg)
.jpg)
.jpg)

Comments
Post a Comment
If you have any doubts or suggestions please kindly share.